
24th European Congress of Psychiatry / European Psychiatry 33S (2016) S72–S115
S101
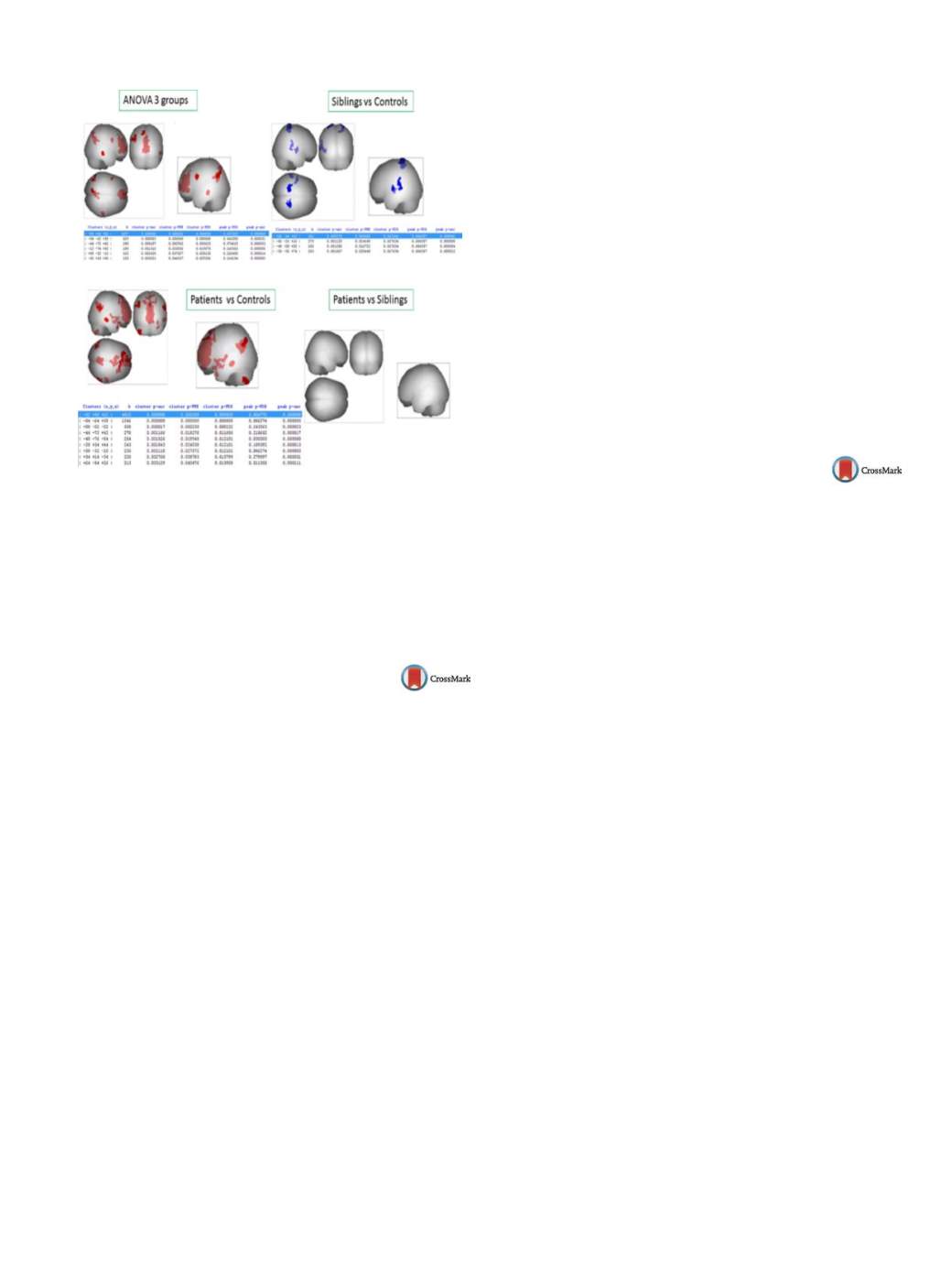
Fig. 1
Disclosure of interest
The authors have not supplied their decla-
ration of competing interest.
Acknowledgments
L. Galindo is funded by the fellowship Río
Hortega Spanish government ISCIII (CM14/00111).
http://dx.doi.org/10.1016/j.eurpsy.2016.01.074FC71
An interventional, multi-center,
randomized, double-blind,
placebo-controlled, active reference,
flexible dose study of brexpiprazole in
adults with acute schizophrenia
S.R. Marder
1, M. Hakala
2 ,∗
, M. Gislum
3, A. Skuban
4, E. Weiller
5,
C. Weiss
61
Desert Pacific Mental Illness Research, Education and Clinical
Center, Semel Institute for Neuroscience at UCLA, Department of
Psychiatry and Biobehavioral Sciences, Los Angeles, USA
2
Lundbeck A/S, ICR Psychiatry, DK, Valby, Denmark
3
H. Lundbeck A/S, Department of Biostatistics, Valby, Denmark
4
Otsuka Pharmaceutical Commercialization and Development Inc.,
Global Clinical Development, Princeton, USA
5
H. Lundbeck A/S, Medical Affairs, Valby, Denmark
6
Otsuka Pharmaceutical Commercialization and Development Inc.,
Global Medical Affairs, Princeton, USA
∗
Corresponding author.
Introduction
Brexpiprazole is a serotonin-dopamine activity
modulator that is a partial agonist at 5-HT
1A
and dopamine D
2
receptors at similar potency, and an antagonist at 5-HT
2A
and nor-
adrenaline alpha
1B/2C
receptors.
Objectives
Evaluating the efficacy, safety, and tolerability of flex-
ible doses of brexpiprazole compared with placebo in patients with
acute schizophrenia.
Aim
Primary endpoint was change from baseline to week 6 in
PANSS total score and key secondary endpoint was change from
baseline to week 6 in CGI-S score.
Methods
Phase 3, multi-center, randomized, double-blind,
placebo-controlled, active reference, trial (NCT01810380). Hospi-
talized patients were randomized to brexpiprazole (2 to 4mg/day),
placebo, or quetiapine extended release (400 to 800mg/day) for
6 weeks. Quetiapine was included as an active reference. Changes
from baseline were analyzed using an MMRM approach.
Results
Mean change in PANSS total score was
−
20.0 and
−
15.9
in the brexpiprazole (
n
= 150) and placebo (
n
= 159) groups, respec-
tively (
P
= 0.056). Sensitivity analyses suggested treatment effect
(e.g., ANCOVA, LOCF:
P
= 0.025; ANCOVA, OC:
P
= 0.026). Mean
change in PANSS total score (
−
24.0) with quetiapine (
n
= 150) was
significantly greater than thatwithplacebo (
P
< 0.001), demonstrat-
ing sensitivity of the assay. Brexpiprazole separated from placebo
on the mean change in CGI-S score (
−
1.2 vs.
−
0.9,
P
= 0.014). The
proportion of patients reporting TEAEs were similar between the
brexpiprazole and placebo treatment groups (54% versus 54.7%).
Conclusion
Treatment with brexpiprazole showed a clinically
meaningful improvement in patients with acute schizophrenia.
While the difference between brexpiprazole and placebo only
approached statistical significance, sensitivity analyses and sec-
ondary endpoints supported a treatment effect of brexpiprazole.
Disclosure of interest
The authors have not supplied their decla-
ration of competing interest.
http://dx.doi.org/10.1016/j.eurpsy.2016.01.075FC72
Are self-stigma and coping strategies
interrelated in outpatients with
schizophrenia spectrum disorders
using the psychiatric medication?
Cross-sectional study
M. Holubova
1 , 2 ,∗
, J. Prasko
1, R. Hruby
3, K. Latalova
1,
M. Slepecky
4, M. Marackova
1, D. Kamaradova
1, T. Gubova
21
Faculty of Medicine and Dentistry, Palacky University Olomouc,
University Hospital Olomouc, Department of Psychiatry, Olomouc,
Czech Republic
2
Regional Hospital Liberec, Department of Psychiatry, Liberec, Czech
Republic
3
Psychiatric Outpatient Department, Psychiatric Outpatient
Department, Martin, Slovakia
4
Faculty of Social Science and Health Care, Constantine the
Philosopher University in Nitra, Department of Psychology Sciences,
Nitra, Slovakia
∗
Corresponding author.
Introduction
Self-stigma is the maladaptive psychosocial phe-
nomenon that can affect the patient’s self-image, may lead to
dysphoria, social isolation, reduced adherence and quality of life.
Maladaptive coping strategies may adversely disturb the overall
functioning of psychiatric patients.
Objectives
Thinking about coping strategies and self-stigma in
practice may play a significant role in understanding patients with
schizophrenia spectrum disorders, especially for mental health
professionals. Focus on coping strategies could be a useful con-
cept in supportive and educational therapy to help patients in using
more adaptive coping strategies and decrease their self-stigma.
Aims
The aimof this studywas to determine the relationbetween
coping strategies and the self-stigma among outpatients with
schizophrenia and related disorders.
Methods
Stress Coping Style Questionnaire (SVF-78), Internal-
ized Stigma of Mental Illness (ISMI) and severity of the disorder
(measured by Clinical Global Impression objective and subjective
form) were assessed.
Results
One hundred and four patients suffering from
schizophrenia (
n
= 67), schizoaffective disorder (
n
= 30), poly-
morphic psychotic disorder (
n
= 3), schizotypal disorder (
n
= 2)
and delusional disorder (
n
= 2) were included in the study. The
results showed that there was a high positive correlation between
negative coping and self-stigma, and the negative correlation
between positive strategies and the overall score of self-stigma.
Stepwise regression analysis showed that negative coping (espe-
cially resignation), subjective severity SubjCGI and positive coping


















